
M+FLO-CO2 Hardness Removal/Neutralization Technology Introduction: Traditionally, water quality hardness softening primarily employs the Ca(OH)₂/NaOH + Na₂CO₃ method, while high-alkalinity water hardness softening predominantly uses the Na₂CO₃ method. These methods not only consume high-cost soda ash but also require the addition of sulfuric acid for subsequent pH neutralization and adjustment, resulting in significant side effects, particularly the introduction of sodium and sulfate ions, which increase water salinity. Additionally, the neutralization and pH adjustment of alkaline wastewater in conventional processes often rely on mineral acids such as sulfuric acid or hydrochloric acid. To meet increasingly stringent environmental and safety standards, as well as cost-effective water treatment requirements, the M+FLO-CO₂ Alkaline Wastewater Hardness Removal/Neutralization Technology has emerged as a trend under carbon neutrality requirements. European legislation now mandates that alkaline wastewater must be neutralized before discharge, with CO₂ neutralization recommended as the preferred process. The PACIF magnetic flocculation + chemical method for hardness removal and neutralization provides an optimized process package and system equipment, including CO₂ automatic injection, detection, efficient hardness removal/neutralization reaction purification, and complete technical equipment (liquefied CO₂ is transported and stored in a liquid CO₂ tank by professional gas companies). The standard process flow is as follows:
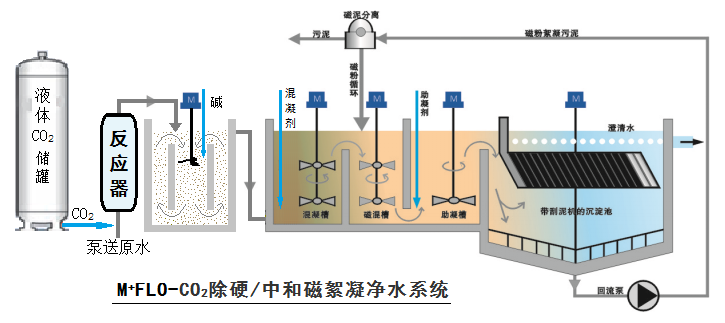
M+FLO-CO₂ Hardness Removal/Neutralization Technology: Principles and Advantages
Chemical Reaction Principles:
CO₂ + H₂O → H⁺ + HCO₃⁻
Ca²⁺ + Mg²⁺ + OH⁻ + HCO₃⁻ → CaCO₃↓ + Mg(OH)₂↓ + H₂O
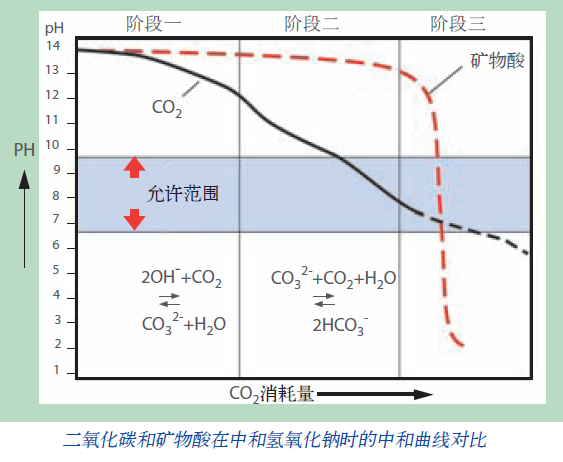
The M+FLO-CO₂ Alkaline Wastewater Hardness Removal/Neutralization Technology offers significant dual advantages in terms of environmental protection and economic efficiency. It features low chemical consumption and a gentle neutralization curve compared to mineral acids. When CO₂ dissolves in water, it forms a weak diprotic acid, with its neutralization performance determined by the pH value of the water. This ensures precise control of the neutralization process without causing over-neutralization issues common with mineral acids. Additionally, it eliminates the risks of equipment corrosion or safety hazards associated with sulfuric acid.
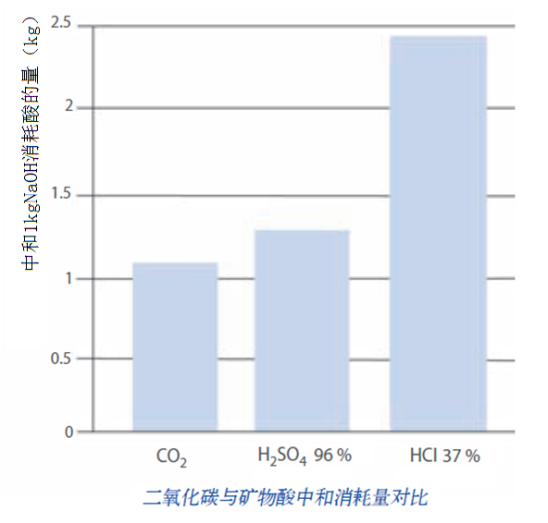
M+FLO-CO₂ Alkaline Wastewater Hardness Removal/Neutralization Does Not Increase Inorganic Salt Content in Water, While the Use of Hydrochloric Acid, Sulfuric Acid, and Similar Agents Cannot Avoid Water Salinization. The M+FLO-CO₂ Process Has a Distinct Advantage in the 'Carbon Neutrality' Direction in Terms of Enhancing Wastewater Discharge Standards and Reducing the Content of Inorganic Salts in Wastewater.
M+FLO-CO₂ Hardness Removal/Neutralization System: PLC Intelligent Control for Automatic Addition and Operation, Simple Operation.
Typical Applications of M+FLO-CO₂ Hardness Removal/Neutralization Technology:
1.Alkaline Wastewater Treatment: M+FLO-CO₂ effectively removes hardness by neutralizing alkaline wastewater, deeply eliminating calcium/magnesium ions, silicon, and other substances. It also efficiently removes heavy metals.
2.Industrial Wastewater Treatment: Combined with the addition of Ca(OH)₂, LDH, NaOH, CO₂, PFS/HFO, and PAM, the M+FLO-LDH/CO₂ system effectively removes sulfate, chloride, silicate ions, and hardness caused by calcium/magnesium ions. It neutralizes alkalinity and adjusts the pH value to the range of 6-9.

Case Study: Ningxia Multi-dimensional Group Biopharmaceutical Wastewater Treatment - High Sulfate and High COD, Pretreatment with Ca(OH)₂ to Generate CaSO₄ Precipitation for Pre-removal of Sulfate and Residual Hardness, Treatment Process:
Raw water → LDH Reactor Tank + M+FLO Magnetic Flocculation System → M+FLO-CO₂ Hardness Removal/Neutralization Magnetic Flocculation System → Effluent
Process Description: In the LDH Reactor Tank, Ca(OH)₂ is added to form gypsum (CaSO₄), which is then purified in the M+FLO Magnetic Flocculation System. The water is further purified and pH adjusted to 7-8 through the M+FLO-CO₂ Hardness Removal/Neutralization Magnetic Flocculation System (with CO₂ addition). Treatment capacity: 6,000 m³/day. Pre-treatment water quality: SO₄²⁻ 2,500 mg/L, COD 6,300 mg/L. Post-treatment water quality: SO₄²⁻ 1,300 mg/L, hardness (as CaCO₃) 90 mg/L. (Note: When Ca(OH)₂ and LDH are added to the LDH Reactor Tank, the sulfate concentration in the effluent can be reduced below SO₄²⁻ 100 mg/L.)
 换一换
换一换