
The chemical method using lime/soda ash for hardness removal results in the formation of calcium and magnesium precipitates, CaCO₃↓ and Mg(OH)₂↓, which exist as fine colloidal particles suspended in water. These particles can adsorb other fine particles, forming a double electric layer that is difficult to coagulate and has poor settling properties. Conventional high-efficiency sedimentation tanks often fail to achieve satisfactory separation, necessitating the construction of large sedimentation basins.
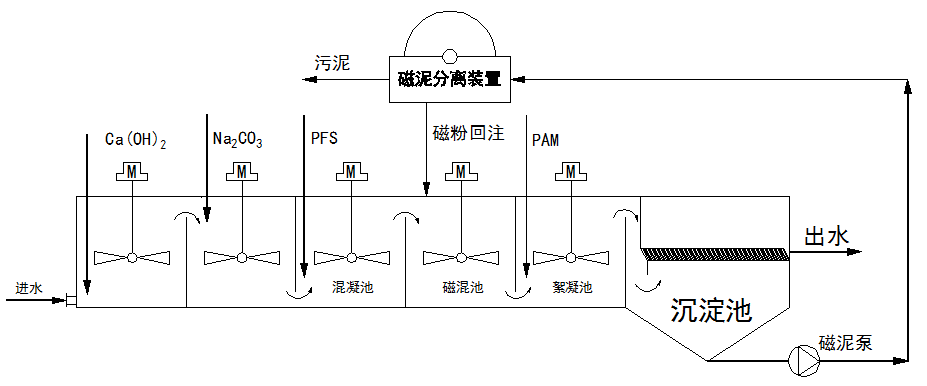
The M+FLO magnetic flocculation sedimentation tank represents a revolutionary upgrade over traditional high-efficiency sedimentation tanks. By introducing magnetic powder into the flocculation process, it forms denser and larger magnetic flocs that can adsorb and coagulate colloidal and other fine particles, achieving deep removal of insoluble particles. The effluent has a residual hardness of less than 0.3 mmol/L, turbidity below 1.0 NTU, and suspended solids (SS) below 3 mg/L. Additionally, this system effectively removes silicon, arsenic, iron, manganese, phosphorus, fluorine, oils, and algae. The magnetic flocs settle rapidly due to the high specific gravity of the magnetic powder (5.0), resulting in a short system retention time of 15 minutes, reduced footprint, and lower engineering investment. The system can be constructed as modular skid-mounted equipment or as concrete tank structures. The process flow is as follows:
[Process flow details would be inserted here based on the specific system design.]
Chemical Reaction Principles of Lime/Soda Ash for Calcium and Magnesium Hardness Removal:
1.Lime (Ca(OH)₂) can remove CO₂ and carbonate hardness (temporary hardness) from water:
CO₂ + Ca(OH)₂ → CaCO₃↓ + H₂O
Ca(HCO₃)₂ + Ca(OH)₂ → 2CaCO₃↓ + 2H₂O
Mg(HCO₃)₂ + 2Ca(OH)₂ → 2CaCO₃↓ + Mg(OH)₂↓ + 2H₂O
Note: NaOH can also be added to adjust pH, producing CaCO₃↓ and Mg(OH)₂↓ to remove temporary hardness.
2.Soda ash (Na₂CO₃) can remove non-carbonate hardness (permanent hardness) from water:
CaSO₄ + Na₂CO₃ → CaCO₃↓ + Na₂SO₄
CaCl₂ + Na₂CO₃ → CaCO₃↓ + 2NaCl
MgSO₄ + Na₂CO₃ → MgCO₃ + Na₂SO₄
MgCl₂ + Na₂CO₃ → MgCO₃ + 2NaCl
MgCO₃ + H₂O (at higher pH) → Mg(OH)₂↓ + CO₂↑
Chemical Dosage and Sludge Discharge Calculation:
Ca(OH)₂ = 37 (CO₂ + A₀ + HMg) mg/L
Na₂CO₃ = 53 Hr mg/L
PFS = 30~100 mg/L
Sludge discharge = 29 HMg + 50 (HCa + CO₂ and A₀) + PFS * 107/278 + ΔSS mg/L
Coagulant aid PAM = 1~2 mg/L
Notes:
A₀: Total alkalinity (in terms of H⁺) mol/L
ΔSS: Influent SS mg/L
HMg: Magnesium hardness mol/L
HCa: Calcium hardness mol/L
Hr: Total permanent hardness (HCa + HMg) mol/L
Typical Applications:
Municipal/Industrial Park Wastewater Treatment: Tailwater used as industrial circulating cooling water, for hardness removal and softening, or as pretreatment for RO desalination membranes (removing calcium, magnesium, silicon, etc.).
Industrial Circulating Cooling Water: Removal of calcium and magnesium hardness for reuse as makeup water, or as pretreatment for RO desalination membranes (removing calcium, magnesium, oil, algae, COD, silicon, SS, etc.).
Industrial Wastewater Zero Discharge: Pretreatment for RO membranes (removing calcium, magnesium, silicon, SS, etc.).
River Water/Mine Water Treatment: Hardness removal and softening for industrial use, or as pretreatment for RO membranes (removing calcium, magnesium, silicon, SS, algae, etc.).
Drinking Water Treatment: Hardness removal and softening for water sources with excessive hardness (removing calcium, magnesium, algae, SS, etc.).
Typical Application Cases:

Qitaihe Coal Mine Wastewater Treatment Project
The wastewater from Qitaihe Coal Mine, characterized by high salinity, is treated at a capacity of 5,000 tons per day. After treatment, the water is reused in the coal-fired power plant of the group. The primary treatment processes include chemical hardness removal/magnetic flocculation + reverse osmosis (RO) membrane desalination. The chemical hardness removal/magnetic flocculation process effectively removes calcium, magnesium, and suspended solids (SS).
Influent Water Quality:
Hardness ≤ 550 mg/L
SS ≤ 150 mg/L
Effluent Water Quality:
Hardness ≤ 50 mg/L
SS ≤ 3.0 mg/L (before membrane treatment)
Note: In cases of high calcium hardness, the IRFR (Internal Recycle Fluidized-bed Reactor) granulation process can be employed. Unlike the traditional method of adding Ca²⁺ to form CaCO₃ precipitates, this process involves the nucleation and growth of CaCO₃ crystals on M⁺ seed particles, producing purer CaCO₃ granules and magnesium sludge, thereby enabling resource utilization.
Typical Applications:
Reuse of treated wastewater in coal-fired power plants.
Chemical hardness removal and RO membrane desalination for high-salinity wastewater.
Utilization of IRFR granulation technology for resource recovery in high-calcium hardness scenarios.

 换一换
换一换